

Home || Research Interests || Publications || Group Members || SOP manuals
Project SEED || MSU Chemistry || MSU
![]()
Bioorganic Chemistry
![]()
The fundamental properties of protein/chromophore interactions can be studied by a rational protein design strategy. Inspired by the example of the visual pigments that give rise to color vision, we have initiated a research program to redesign a set of small,soluble, stable and easily manipulated retinoid binding proteins to bind the retinal chromophore covalently as a protonated Schiff base (PSB). The Cellular Retinoic Acid Binding Protein II (CRABPII) and the Cellular Retinol Binding Protein II (CRBPII) are chosen from the intracellular lipid binding protein family (iLBP) since the structures of these proteins remain stable even when substantially mutated. Additionally, their large interior binding cavities allow us to apply a wider range of design strategies and can accommodate a broad range of chromophores. The resulting novel protein/chromophore complexes exhibit a wide variety of colors over the entire visible region of the electromagnetic spectrum.

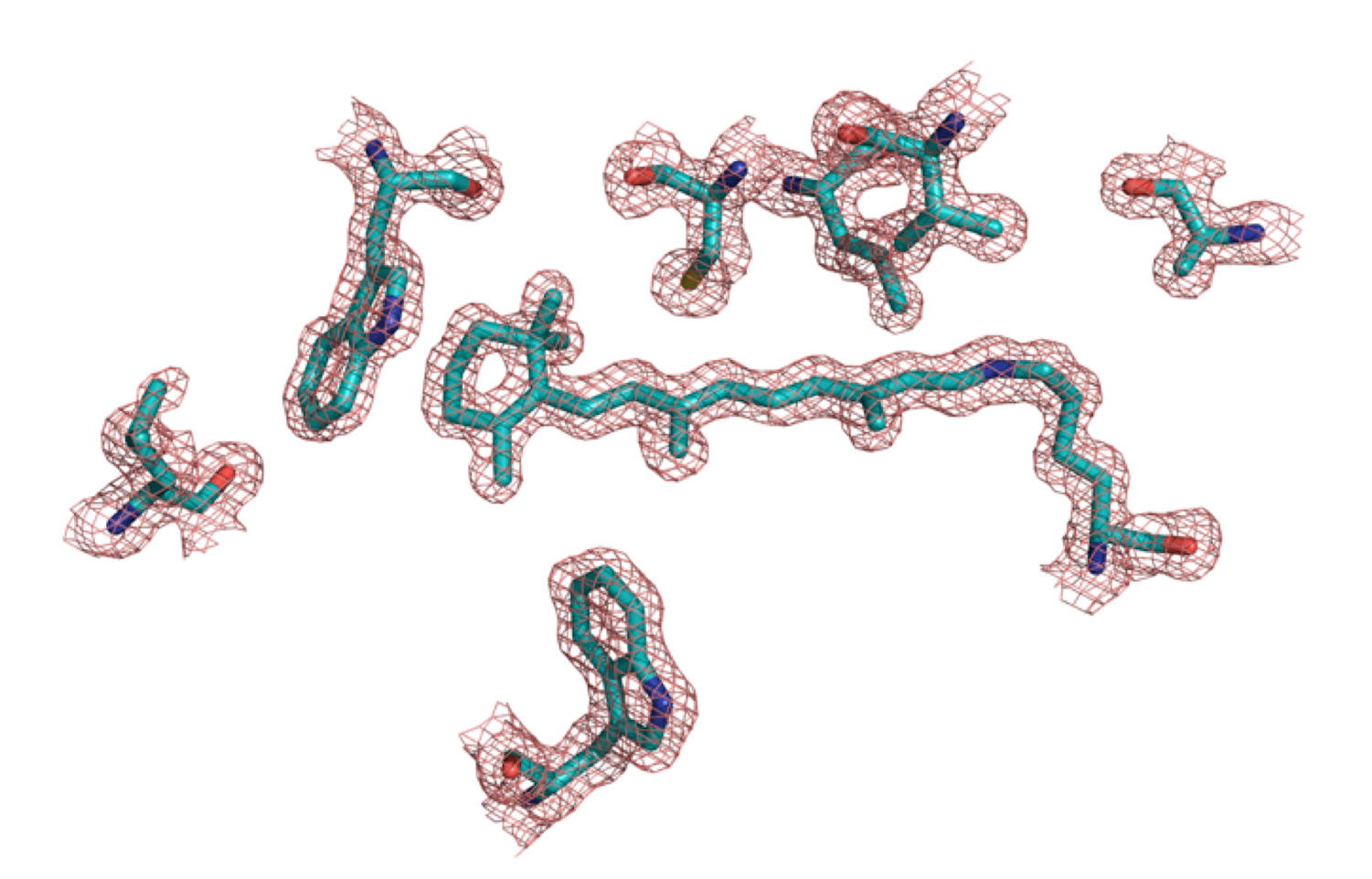
We are exploring the potential to use our engineered proteins as colorimetric and fluorescent proteins for various applications. As a first step in achieving this goal, the merocyanine aldehyde derivative with a resonating enamine-iminium system was synthesized and complexed with our engineered mutants. The study has yielded a variety of chromophoric and fluorophoric proteins that can target various organelles, if tagged with the appropriate localization signal.
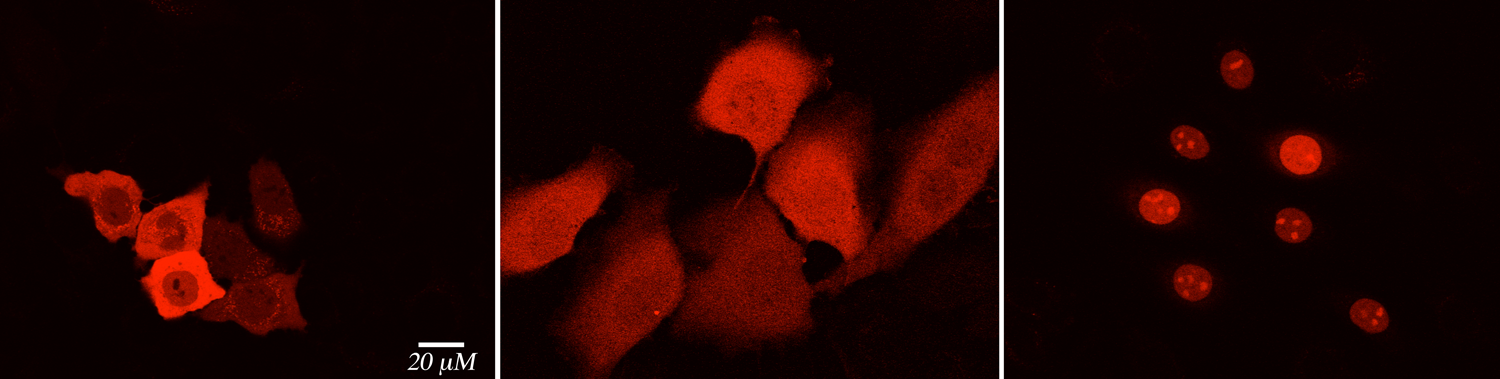
Intracellular pH sensors would be a valuable addition to the arsenal of protein-based sensors. Small molecule pH sensors are used commonly, however, they are not capable of targeted delivery to various organelles. We are exploring the use of our engineered proteins to design novel protein-based pH sensors. We have discovered new CRABPII mutants that are amenable to modulation of the PSB's pKa, yielding engineered proteins that are colorimetric at various pH ranges. This feature makes these mutants uniquely suited to function as pH sensors with further modifications as fluorescent pH-sensing tags.

The Conantokins are short peptides found in the venoms of predatory sea snails and act as antagonists of the NMDA receptor. One of the members of this family, Con-G, has the interesting property of becoming helical only in the presence of divalent metal ions such as calcium and magnesium. In addition, while Mg2+ binding results in helix monomers, Ca2+ binding results in helix dimerization. This and similar peptides have come to be known as "metallo-zippers" and in many ways they resemble the well-known "leucine-zippers" due to their propensity to form α-helical structures, and their ability to dimerize. Although much less is known about metallo-zippers, they promise significant advantages as compared to other helix-formers, due to their ability to form helical structures upon addition of divalent cations, the ability to exquisitely control their level of oligomerization via both changes in sequence and cation, and the lack of any hydrophobic interactions in their interface. The stage is set for a workman-like approach to better understand the structural determinants for folding and oligomerization.
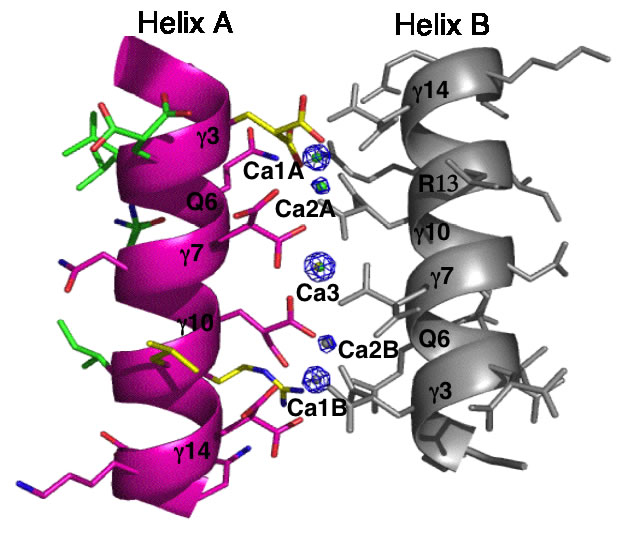
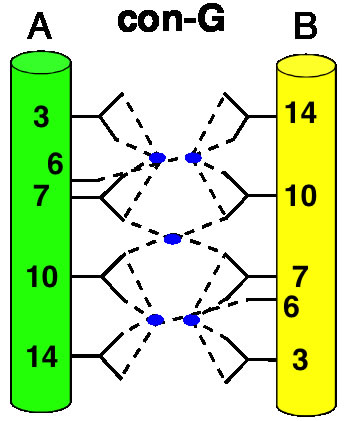
![]()
Recent Publications
![]()